The Dr. Manya HCV Antibody Test Kit (Pack of 25) is a scientifically designed diagnostic tool for the qualitative detection of Hepatitis C Virus (HCV) antibodies in human blood, serum, or plasma. It is widely used in preventive healthcare, mass screening programs, clinical diagnostics, and public health awareness campaigns.
This kit empowers users to screen individuals for Anti-HCV antibodies, which are produced by the body in response to Hepatitis C infection. With an easy-to-use design and results in 15-30 minutes, it supports early identification and clinical decision-making, especially in resource-limited settings.
What Are Anti-HCV Antibodies?
When a person is exposed to the Hepatitis C virus, their immune system produces antibodies—proteins that help fight off infections. The presence of these antibodies in a blood sample suggests:
-
A current or past infection with HCV
-
The need for further diagnostic tests like HCV RNA PCR to confirm an active infection
Why Is Hepatitis C Testing Important?
-
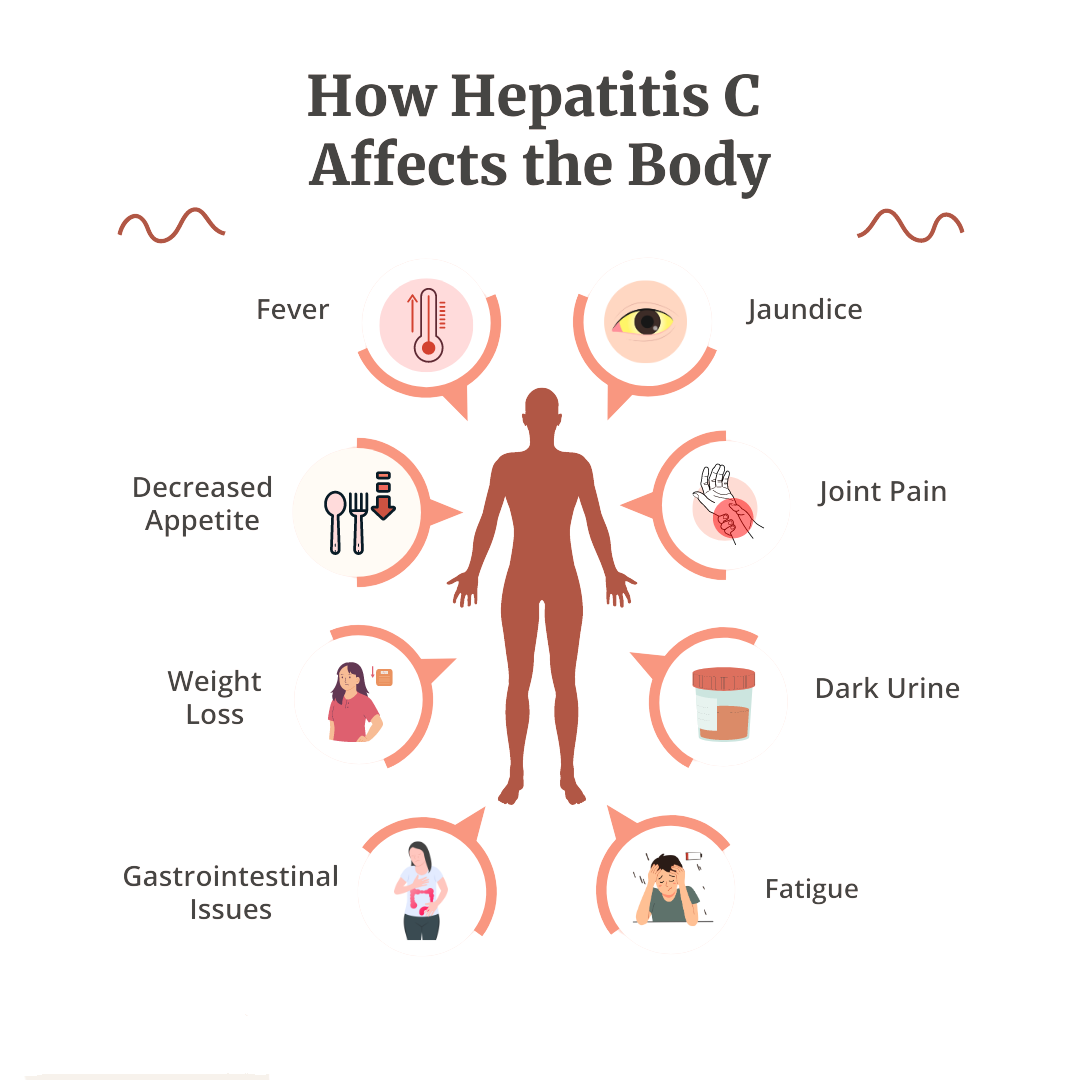
Hepatitis C is a bloodborne virus that causes liver inflammation and, over time, can lead to chronic liver disease, cirrhosis, and liver cancer.
-
Many infected individuals show no symptoms for years, making routine screening essential—especially for high-risk populations.
-
Early detection allows timely treatment with antiviral medication, which can cure over 95% of cases when identified early.
Who Should Be Screened?
This test kit is particularly suitable for:
-
Healthcare workers and first responders
-
Patients with a history of blood transfusions or organ transplants (pre-1992)
-
People with IV drug use history
-
Individuals with HIV or other liver conditions
-
Family members of HCV-positive individuals
-
Adults aged 18–79, as recommended by global health agencies for one-time screening
Key Features & Benefits:
| Feature | Description |
|---|---|
| Test Type | Rapid Lateral Flow Immunoassay |
| Detects | Anti-HCV Antibodies (IgG/IgM) |
| Result Time | 15-30 Minutes |
| Sample Type | Whole Blood, Serum, or Plasma |
| Storage Conditions | 2°C to 30°C (Room Temperature) |
| Shelf Life | 24 Months from MFG Date |
| Use Case | Bulk Testing, Screening, Field Use |
| Certifications | CE Marked, ISO |
What’s Inside the Pack (25-Test Kit):
-
25 x HCV Antibody Test Cassettes
-
25 x Sample Droppers
-
Buffer Solution
-
Detailed Instruction Manual
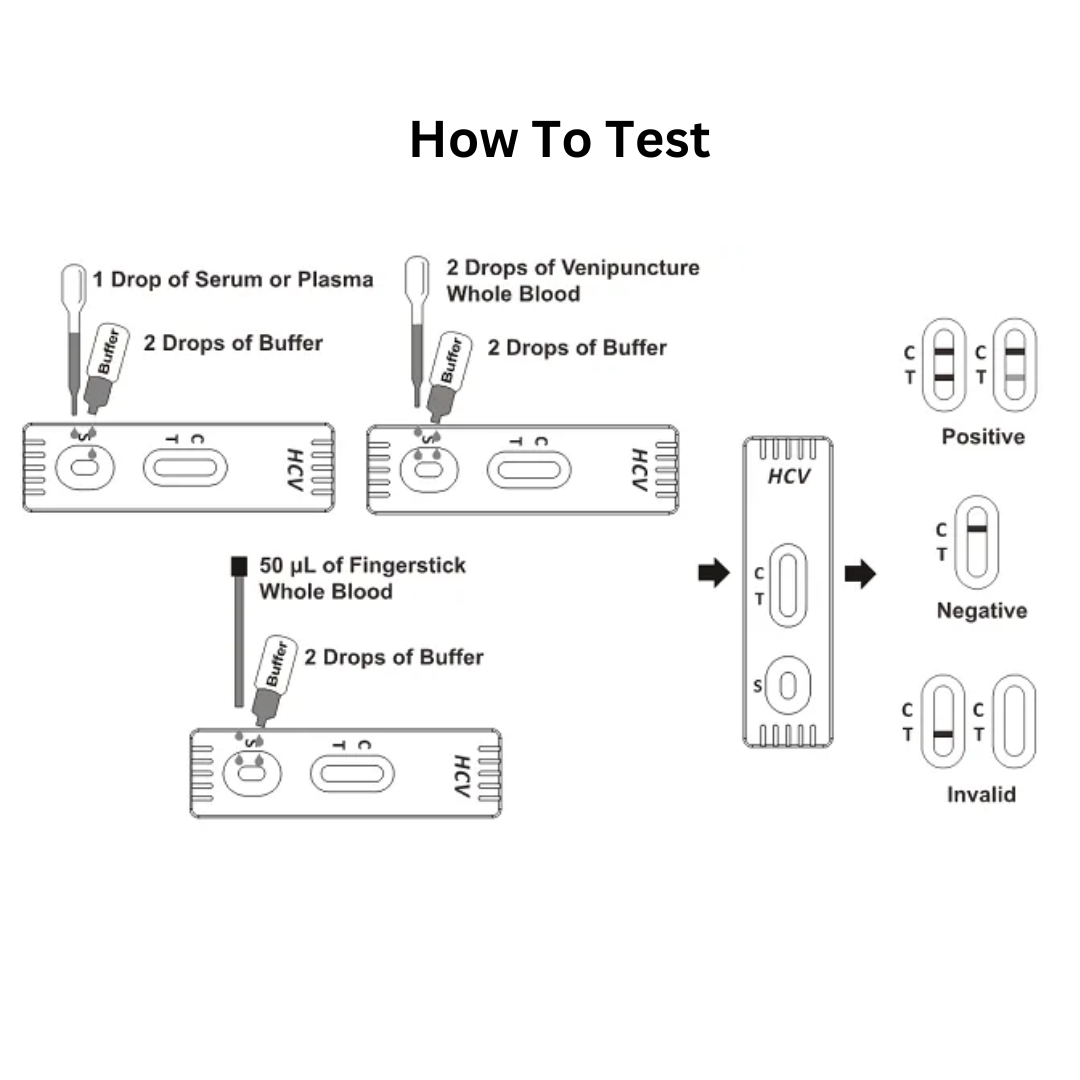
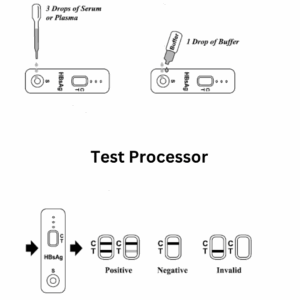
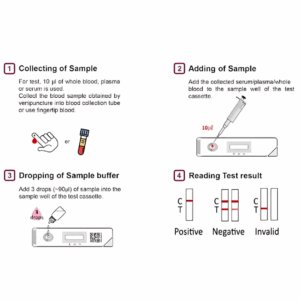
How to Perform the Test – Step-by-Step Guide:
- Collect a drop of blood with the disposable dropper.
-
Place it on the test cassette.
-
Add 2–3 drops of buffer solution to the designated area.
-
Wait 15-30 minutes and interpret the result:
| Result | Interpretation |
|---|---|
| One line (Control “C”) | Negative for HCV antibodies |
| Two lines (Control “C” and Test “T”) | Positive – HCV antibodies detected |
| No lines or Test line only | Invalid – Retest required |
When to Refer for Further Testing
If the test result is positive, it indicates exposure to the Hepatitis C virus, but not necessarily an active infection. To confirm, the following tests are recommended:
-
HCV RNA PCR Test – Detects the actual presence of viral RNA
-
Liver Function Tests (LFTs) – Assess liver damage
-
Genotyping Test – Determines the specific HCV strain for treatment planning














 No products in the cart.
No products in the cart. 
Reviews
There are no reviews yet.